How is MONJUVI different from other treatments?
Understanding your FL
Follicular lymphoma (FL) is a type of cancer that affects the lymph nodes, bone marrow, and other organs. It is incurable and tends to grow more slowly than other types of cancer.
Relapse is a common part of the FL journey, and while it may feel discouraging, it’s important to remember that remission may still be within reach. That’s why working closely with your doctor to choose the next treatment that’s right for you is so important. MONJUVI in combination with rituximab and lenalidomide is an option that offers a chance to live longer without disease progression.
MONJUVI is not chemotherapy
MONJUVI is a targeted immunotherapy that works with your immune system to help find and kill cancer cells.
How MONJUVI works
B cells are part of a person’s immune system. They help your body fight infection. In FL, B cells grow out of control, both in size and number. MONJUVI targets cancerous B cells directly and activates your immune system to fight FL. MONJUVI can also affect healthy B cells. The image below illustrates how MONJUVI works.
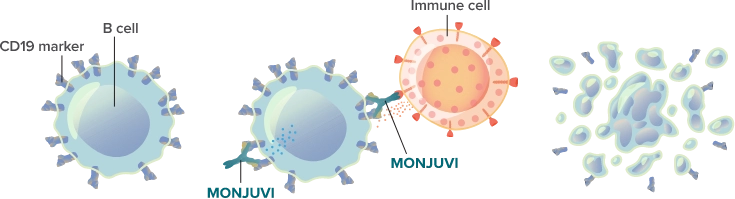
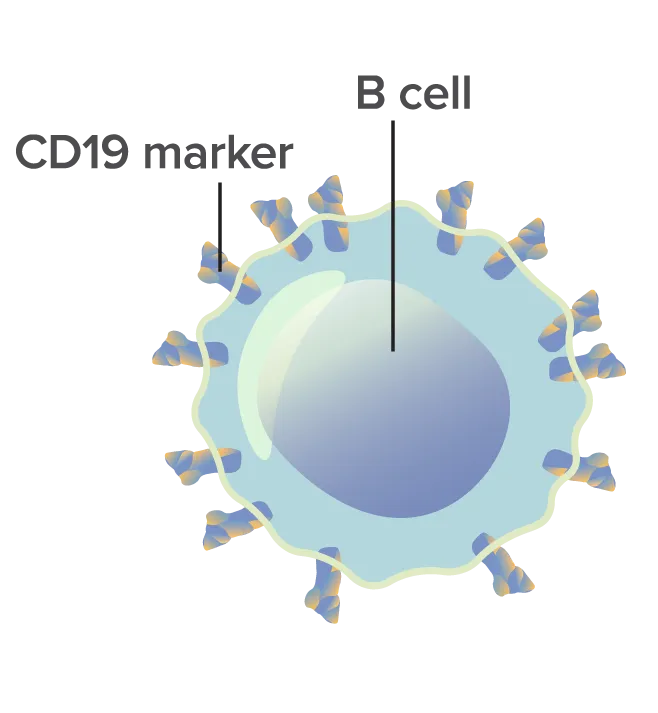
Cancerous B cell with CD19 markers.
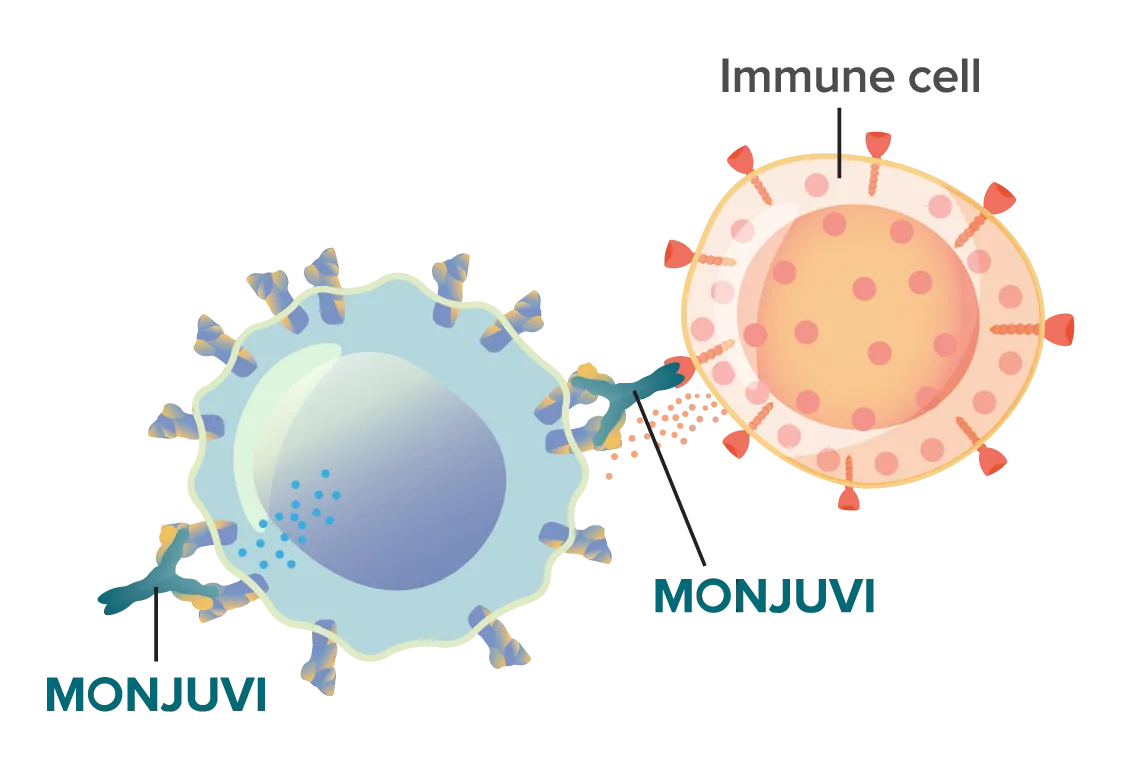
MONJUVI locates and binds to the CD19 marker on the surface of cancerous B cells.
This binding action triggers immune cells to kill the cancer cells.
Why MONJUVI?
MONJUVI was studied in 548 adults whose FL returned, got worse, or didn’t respond to treatment. There were 2 different treatment groups in the study, and both groups received treatment for up to 12 months.
- Group 1: 273 people received MONJUVI in combination with rituximab and lenalidomide
- Group 2: 275 people received placebo in combination with rituximab and lenalidomide
A placebo is a substance with no active ingredient. How your condition progresses and how you may respond to MONJUVI depends on your individual circumstances.
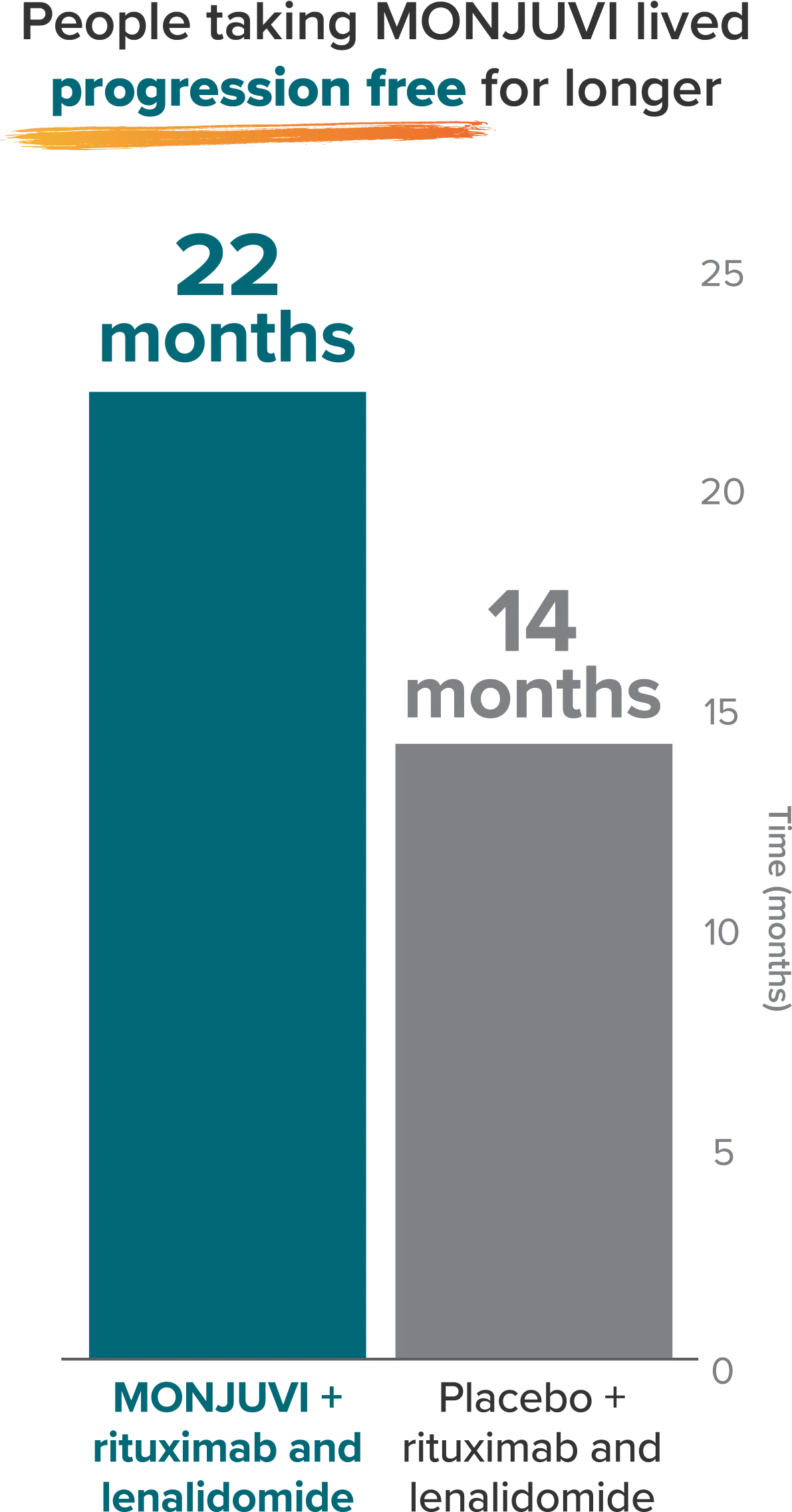
A chance to live longer without disease progression
After a 14.1-month median follow-up in the clinical study, MONJUVI + rituximab and lenalidomide lowered the risk of disease progression or death by 57% compared to placebo + rituximab and lenalidomide.
Median progression-free survival (PFS)
Median PFS is defined as the length of time after the start of study treatment in which half of the people in a treatment group were alive without their disease getting worse (disease progression).

A chance to reach remission
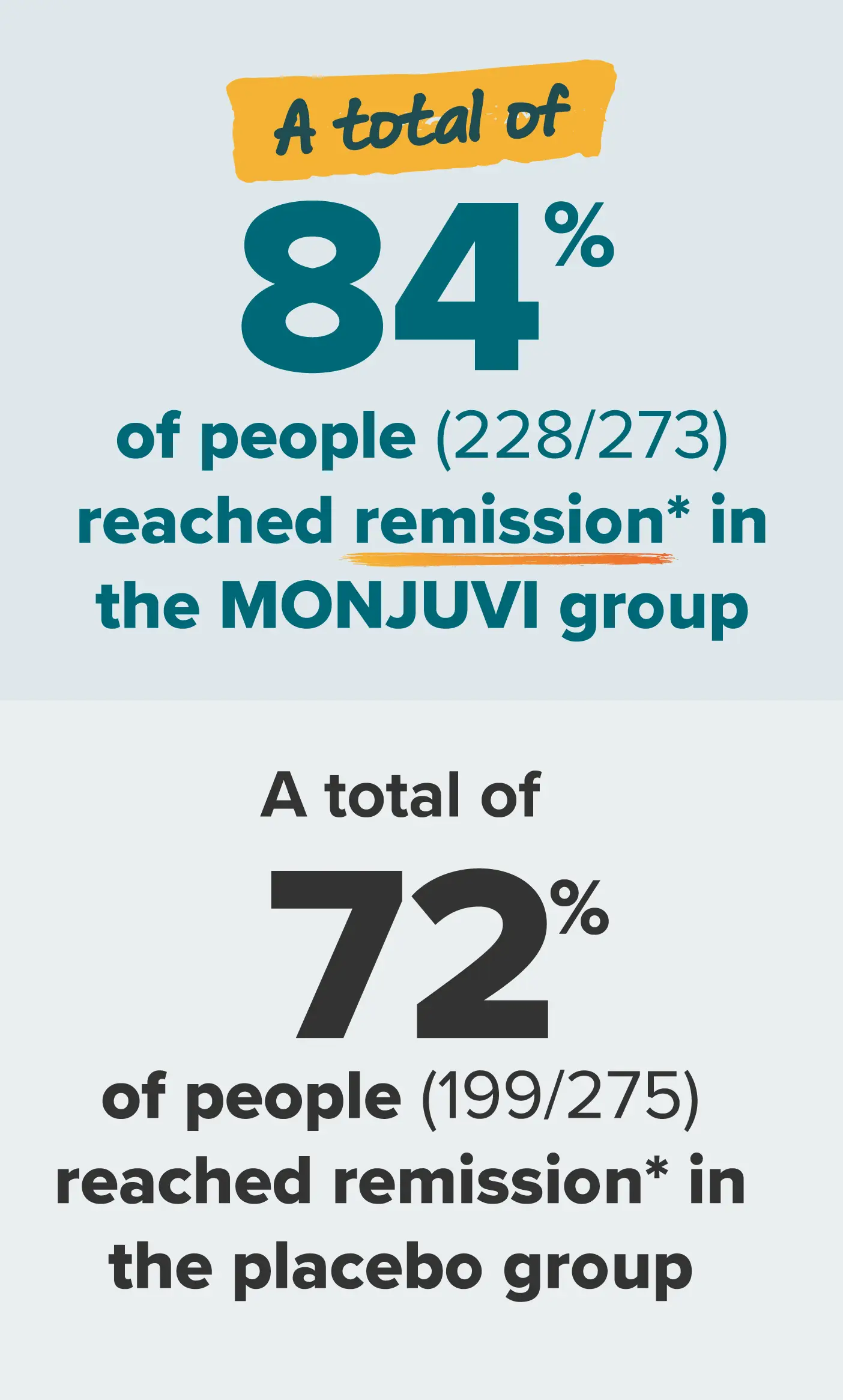
*People reached either complete or partial remission.
Complete remission: the disappearance of all signs of cancer in response to treatment. This does not always mean the cancer has been cured.
Partial remission: a decrease (usually at least 50% in FL) in the size of a tumor, or in the extent of cancer in the body, in response to treatment.
Time to next treatment
In the clinical study, time to next treatment was defined as the time from the start of study treatment to the start of a different, or “next,” treatment for FL for any reason.
For people who received MONJUVI + rituximab and lenalidomide,
there was a 78% chance of not starting a different FL treatment at 2 years after the start of study treatment
For people who received placebo + rituximab and lenalidomide, there was a 57% chance of not starting a different FL treatment at 2 years after the start of study treatment.
During the study, the decision to start a person on a next treatment was based on their doctor’s clinical judgment. The data shown above are not conclusive and should be interpreted with caution. Talk to your doctor if you have any questions.

